BBDRisk Dx® Test in Treating ATYPICAL AND NON-ATYPICAL Breast Growths
Decisional Dilemma in Administering Preventive Therapies
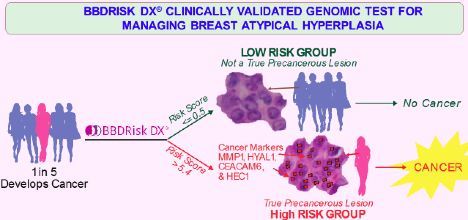
Studies have shown that 20-25% of patients with atypical hyperplasia diagnosis subsequently develop cancer. Currently a number of therapies are available for the prevention of breast cancer for women at increased risk. These include chemoprevention therapy with Tamoxifen (Tam), Raloxifene, or an aromatase inhibitor (AI), and/or surgical therapy with removal of both breasts. However, the histology based diagnosis conferring the increased risk cannot distinguish the ~20-25% of highest risk patients from the ~75-80% of low risk patients. Therefore, patients as well as their providers are faced with a decisional dilemma as to whether or not to proceed with preventive therapy. The uncertainty surrounding risk places an enormous emotional burden on both patients and their physicians.
As a result, patients who are at low risk are unnecessarily subjected to the potential side effects of the above drugs (such as pulmonary embolism, deep vein thrombosis, stroke, cataracts and vasomotor instability (Tam and Raloxifene), endometrial cancer (Tam), or musculo-skeletal pain and bone loss (AIs)) or possibly unnecessary mastectomies. On the other hand, patients who are at elevated risk but choose not to undergo cancer preventive therapy because of uncertainty as to their real risk are not receiving the benefit of life saving prophylactic treatments.
Risk Score by BBDRisk Dx® Test and Medical Management of atypias and other hyperplasias
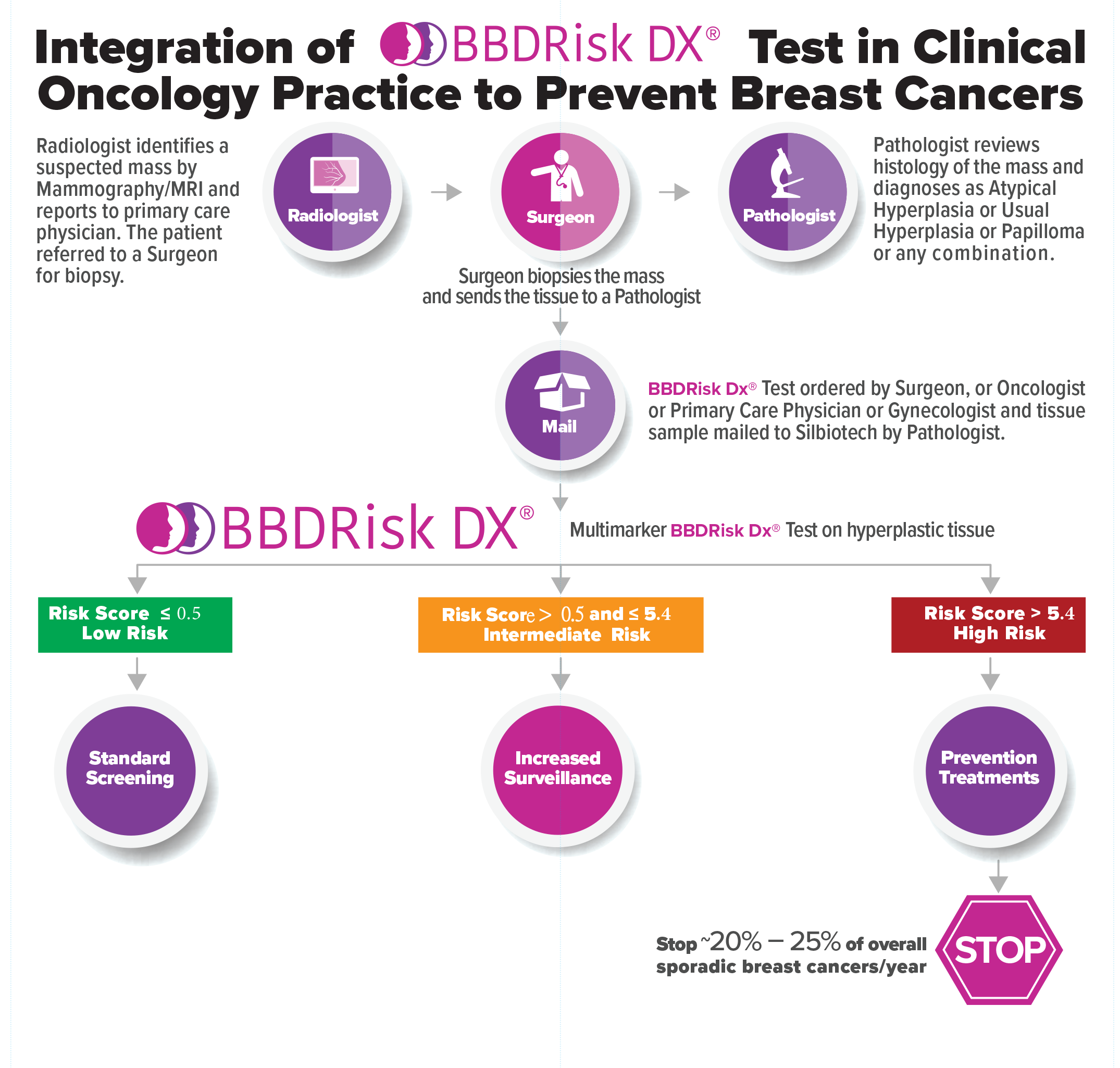
The tumor biology based BBDRisk Dx® genomic test will assess multiple markers in the abnormal tissue and generates a ‘Cancer Risk Score’ in the range of 0-10 with higher scores corresponding to higher risk of subsequent cancer development. The test categorizes the patient as ‘Low Risk’, ‘Intermediate Risk’ or ‘High Risk’ (Figure) by comparing the risk score of a patient with the plurality of scores in Silbiotech’s data base. The test Results Report will also provide the ‘Cancer Free Rate or Cancer Rate’ among subjects who have a similar ‘Risk Score’ from the test validation study. (Click the Sample BBDRisk Dx® Test Result Reports link to open and use the Brower’s File Save As to save it)
BBDRisk Dx® Guides in Informed Medical Management Decisions
Informed medical management decisions can be guided by the ‘Cancer Risk Score’ and ‘Risk Level’ of a patient. BBDRisk Dx® provides molecular evidence to stratify and select those individuals at elevated risk, thereby personalizing treatment and minimizing unnecessary side effects to the low risk candidates. Thus the BBDRisk Dx® test (Flow Diagrams below) provides a standardized modality to assess the risk of an individual with a hyperplastic biopsy demonstrating that she is at increased risk of developing breast cancer. By providing the personalized molecular risk information, patients can be informed of the benefit of prophylactic treatment and are more likely to comply with the treatment recommendation by their healthcare provider.
Medical management decisions can now be made based on the ‘Cancer Risk Score’ and consultation between each patient and his or her healthcare professional.
For example, standard screening for women whose BBDRisk Dx® results indicate a low risk score. If BBDRisk Dx® results indicate that a patient has an intermediate or high risk score, the following medical management options could be applied:
- Increased surveillance
- Risk-reducing medications and/or
- Prophylactic surgery
Disclaimer: Any discussion of medical management options provided here are for general informational purposes only and should not be interpreted as recommendations by Silbiotech, Inc. While BBDRisk Dx® test results provide useful information, medical management decisions should be made based on consultation between each patient and his or her healthcare professional. The patient and his or her doctor are solely responsible for making any medical management decisions.