Hyperplasia Breast Cancer Risk Information
Women diagnosed with breast atypical and non-atypical hyperplasias have an increased risk for breast cancer.
Breast Cancer is the most diagnosed cancer for women in the USA. One in eight women develops breast cancer in her life time. Every year approximately 800,000 to one Million women in the USA undergo breast biopsy for a suspected mass of which ~200,000 to 230,000 are cancerous and the rest are non-cancerous. Of the non-cancerous masses, women diagnosed with Atypical Ductal Hyperplasias (ADH), Atypical Lobular Hyperplasia (ALH), Usual Ductal Hyperplasias (UDH), Papilloma and Sclerosing Adenosis types of masses have an increased risk of developing breast cancer.
One in five women diagnosed with atypical hyperplasias develops breast cancer within 5 or more years
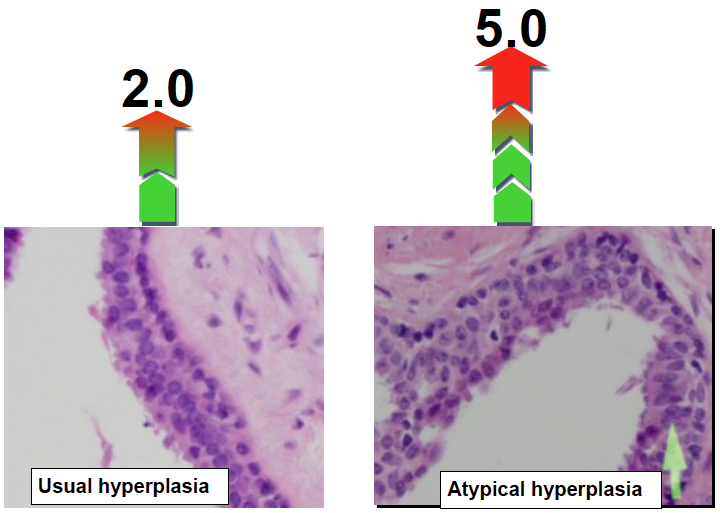
Studies have shown that about 20% of women who are diagnosed with either atypical ductal hyperplasias or atypical lobular hyperplasias subsequently develop cancer within 5 or more years (References 1-17). The question has been how to identify those 20% of women who will go on to develop cancer from the 80% of those who will not develop cancer.
One in ten women diagnosed with non-atypical hyperplasias develops breast cancer within 5 or more years
Studies have shown that women diagnosed with Usual Ductal Hyperplasias, Sclerosing Adenosis, Papilloma or a combination of any of these types have a two-fold increased risk of subsequently developing breast cancer. Overall about one in ten women develops breast cancer within five or more years (References 1-17) (Figure below) after diagnosis of these abnormal growths. Until now, there have not been any tests to identify the 10% of women who will go on to develop breast cancer.
Different benign masses have different risk levels. Usual hyperplastic tumors have a two-fold increased risk and atypical tumors have a five-fold increased risk of subsequent breast cancer development
Testing hyperplasia mass for breast cancer risk
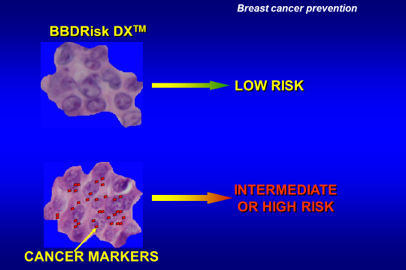
A New Genomic BBDRisk Dx® Test is now available to assess risk
Silbiotech, Inc. offers for the first time a new genomic test, BBDRisk Dx®, for women diagnosed with atypical or usual hyperplasias, sclerosing adenosis, papillomas or a combination of any of these types of abnormalities, to assess the level of risk for developing breast cancer. The test assays a unique set of cancer marker proteins in the biopsied tissue and predicts the likelihood of developing breast cancer (Figure below) for making informed treatment decisions.
BBDRisk Dx® Test Insurance Coverage
BBDRisk Dx® Test coverage is approved by Medicare and most commercial insurance agencies.
REFERENCES
1. Siegel, R., Miller, K.D and Jemal, A. (2017), Cancer statistics, 2017. CA: A Cancer Journal for Clinicians, 67: 7–30.
2. Tavassoli, F. A and Norris, H.J (1990), A comparison of the results of long term follow up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer, 65, 518-29.
3. Foote FW, Stewart FW. Comparative studies of cancerous versus noncancerous breasts. Annals of Surgery.1945; 121:197-222.
4. Ryan JA, Cody CV. Intraductal epithelial proliferation in the human breast- a comparative study. Cancer J/ Surge.1962; 5:2-8.
5. Wellings SR, Jenson HM, Marcum RG (1975) An Atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J. Natl.Cancer Inst. 55, 231-273.
6. Black MM, Barclay TH, Cutler SV, et al. Association of atypical characteristics of benign breast lesions with subsequent risk of breast cancer. Cancer. 1972;29:338-43.
7. Dupont WD, Page, DL. Risk factors for breast cancer in women with proliferative breast cancer. N. Engl. J. Med. 1985;312:146-51.
8. London SJ, Conolly JL, Schnitt SJ, et al. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267:941-4.
9. Dupont WD, Parl FF, Hartman WH Breast cancer risk associated with proliferative breast disease and Atypical hyperplasia. Cancer, 1993;71:1258-65.
10. Page DL, Dupont WD. Association indicators (histologic and cytologic) of increased breast cancer risk. Breast Cancer Research and Treatment. 1993;28:157-166.
11. Karpus CM, Leis HP, Oppenheim A, et al Relationship of fibrocystic disease to carcinoma of the breast. Ann. Surg. 1995;162:1-8.
12. Allerd DC, Mohsin, SK, Fuqua, SAW. Histological and biological evolution of human premalignant breast disease.Endocrine Related Cancer 2001;8:47-61.
13. Krishnamurthy S, Sneige N. Molecular and biological markers of pre-malignant lesions of human breast. Adv. Antomical Pathology 2002;9:185-197.
14. Guray M, Sahin AA. Benign breast diseases: Classification, diagnosis, and management. Oncologist. 2006;11;435-449.
15. Hartman L, Sellers TA, Frost MH, Lingle WL, Frhmom SC, Ghosh K, Vierkant R A, Shaun MAS, et al. Benign breast disease and the risk of breast cancer. New England J. Med. 2005;353: 229-237.
16. S. B. Coopey, E. Mazolla, J.M. Buckley, John Sharko et al The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res. And Treatment, 2012: 10549-012, 2318
17. Worsham M J, Abrams J, Raju U, Kapke A, Lu M, Cheng J, Mott D and Wollman S. R. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007; 13: 116-121.